
Embark on an Adventure: Let’s Explore New Spaces on the Molecular Level
“One small step for man, one giant leap for mankind” – Neil Armstrong
Humans have and will always be explorers. From accessing remote lands on earth to exploring space, Humans are curious and will always try to set foot where no one has ever been before. But we don’t need to go as far as the outer-space to access new inaccessible places: we can just look at the nanoscopic world around us! Put your lab coat and cosmonaut helmet on and let’s show Neil Armstrong a few new places to plant his flag!
Difficulties of exploring the 3D-world on the molecular level
Molecules are an assembly of different atoms (such as Carbon, Hydrogen, Nitrogen or Oxygen for examples) sharing electrons. There are molecules that are planar (2D), and others that exist in 3 dimensions. A lot of research has already been done to explore the spaces on 2D-molecules. But when we add an extra dimension, things tend to become more complicated…
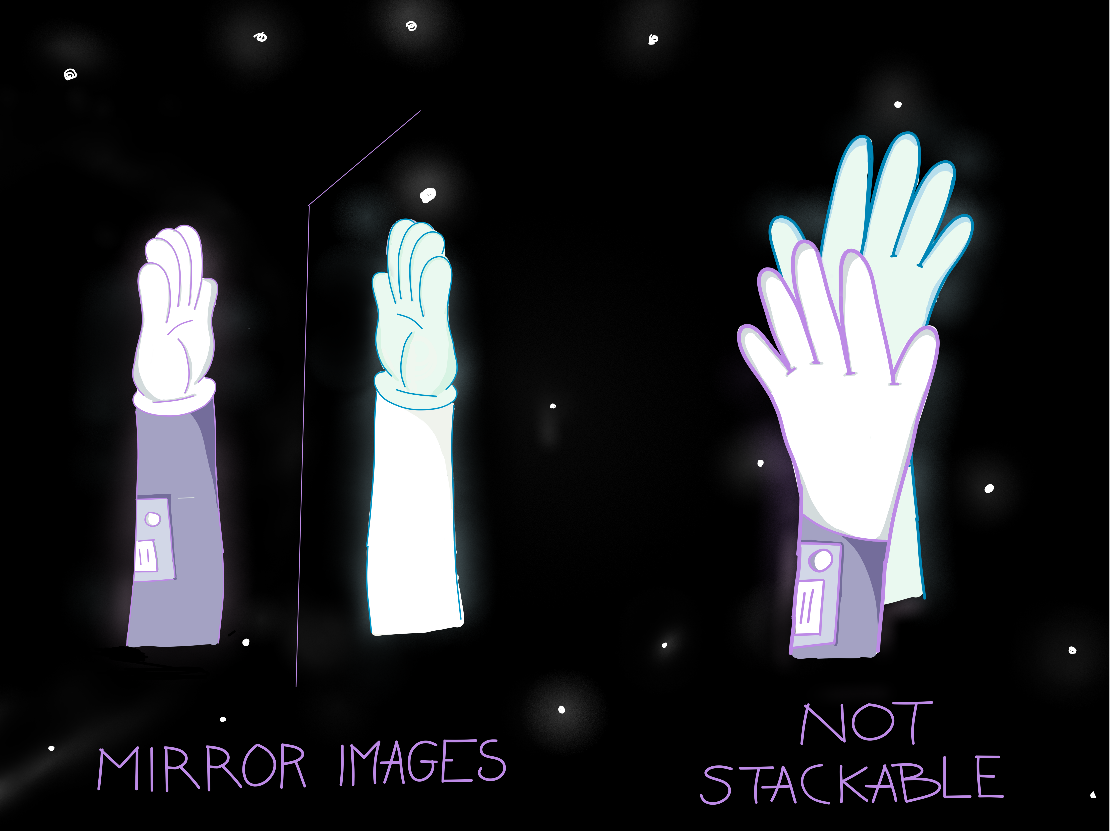
3D-molecules can look very similar, have the same atoms in the same order, and still have different properties. This is what chemists call enantiomers. Enantiomers are mirror-image molecules that can’t be stacked on top of each other. Like our hands! We have the same fingers on both hands, in the same order (thumb next to the index finger, which is next to the middle finger etc…). Our hands are images of each other in a mirror but can’t be stacked on top of each other. Consequently, our hands don’t show the same abilities: we will be able to write with the right hand while the left one is clumsier, for example.
Small Difference, drastic impact
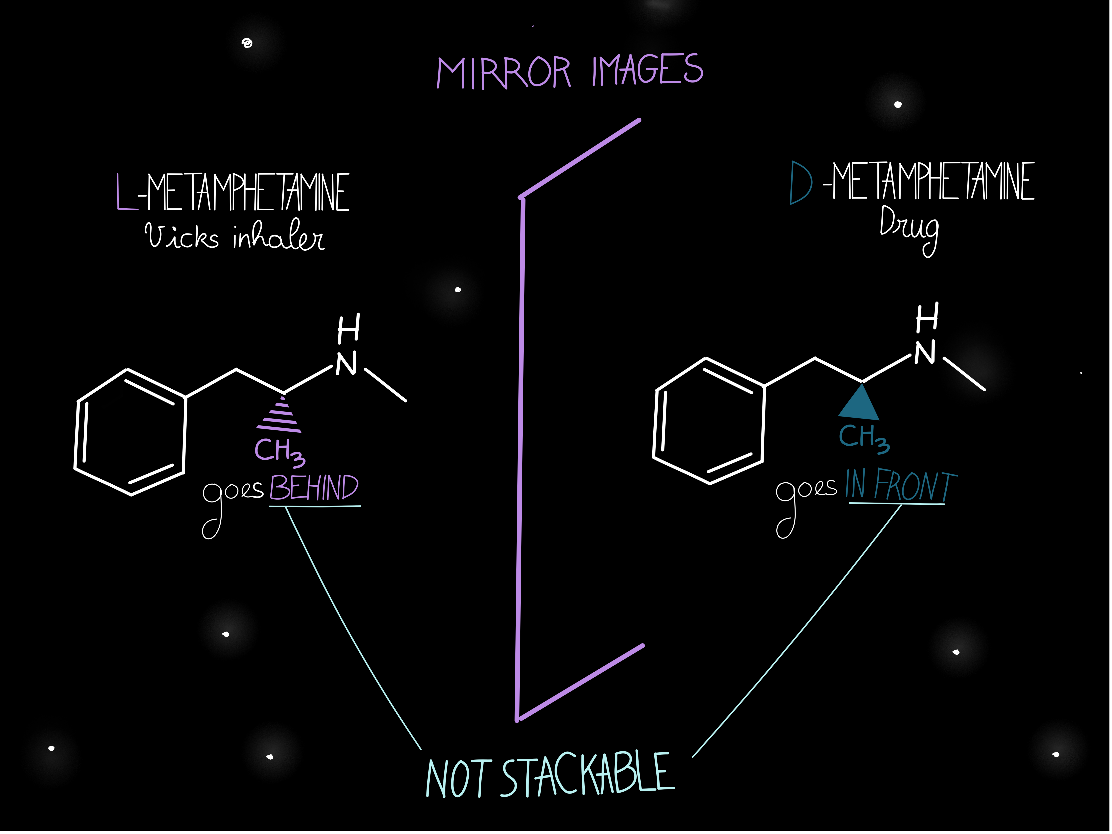
This small geometrical difference can also drastically impact the properties of molecules! For example, methamphetamine can exist as two different enantiomers. One (the right hand) is the active compound of Vicks Inhaler, an over-the-counter medicine used to clear your nose when you have a cold. The other enantiomer (left hand) is an illicit drug. This small geometrical difference has a huge impact on how our body is reacting to the two molecules! Therefore, Vicks has to be extra careful so that the medicine they are selling is in the right form and will produce the desired effect. You would not want to be high on a hard drug after taking medicine for a cold!
But how does Vicks manage to produce only the right enantiomer and not the illicit drug? Two main methodologies can be used by the company to synthesize the desired enantiomer:
- Vicks could use traditional synthetic methodologies where there is no control over which enantiomer is formed preferentially. They would obtain both enantiomers in a 50/50 ratio, which would then need to be separated. This separation is extremely difficult due to the structural similarities of the two molecules and is also going to generate a lot of waste, as they are going to discard D-methamphetamine (or maybe open another business on the side – Breaking Bad, anyone?) to only keep L-methamphetamine.
- Vicks could use a more recent method, where only one enantiomer is formed selectively using a chiral catalyst. A chiral catalyst is a molecule which is going to “block” one face of the molecule and allow the reaction to happen either on the “front” or on the “back” of the molecule and therefore create one enantiomer preferentially. This is what we call enantioselective synthesis. This is what my research is about!
Organocatalyst, a cleaner fuel to explore new chemical spaces
In the past, most of the chiral catalysts used were metal species, but these catalysts come with several drawbacks. First, we only have a limited amount of rare metals on earth. This problem has been particularly highlighted recently with the Russian-Ukrainian conflict, which is greatly impacting the price of these rare metals. Finally, metals pollute – not only our environment but also our body. If a metal catalyst is required during the synthesis of a drug, all traces of metal species must be removed from the drug before giving it to a patient, which is extremely complicated and time consuming.
In the last decades, an alternative has been found and rewarded by the Nobel prize in 2021: organocatalysis. This time, we are not using metal species but small organic molecules (such as proline for example). In comparison to metal catalysts, organocatalysts are abundant on earth, less toxic and cheaper. In my research I am using a special type of organocatalyst containing Iodine.

3D Fluorinated molecules, the ultimate exploration goal
After that, rockets and spacesuits will no longer be required to have our head in the stars!
We have our goal (to access 3D molecules and synthesize one enantiomer selectively), we have our team of astronaut/chemist, we have the rocket filled with green fuel (organocatalyst). Where should we go now? What planet should we explore?
Have you ever heard of fluorine containing molecules? Probably not, and yet I am 100% sure you are consuming fluorinated molecules daily. If you have ever used a Teflon pan, took a drug, or gardened, you are likely to have been using fluorinated molecules, as more than 25% of the pharmaceuticals and 40% of agrochemicals contain at least one fluorine atom.
In most cases, these fluorinated molecules are still in 2D. The role of the fluorine atom is to change the physical/chemical properties of the 2D molecules to make them more efficient. For example, the presence of fluorine is going to make it easier for the fluorinated drug to go through the membrane in our bodies, allowing it to reach the desired site of action faster and be quickly active.
In 3D molecules, fluorine is going to have an additional role. Usually, for a drug to be active, it needs to interact with a receptor in our body, just like a key is going to interact with a lock to open a door. The receptors (locks) are sensitive to the geometry of the molecule (key). Only one key can unlock the door, and only one enantiomer can activate the receptor. Therefore, a big goal of chemical companies and of my research today at the WWU is to finally be able to access and control 3D fluorinated molecules with the help of our iodine organocatalyst. These 3D molecules, which were still unexplored spaces until now, could be the high-value added molecules/keys of tomorrow!

