Can We Transform Alkenes? How Photochemistry Could Help Treat Cancer & Acne With the Same Molecule
Are you curious to find out what photochemistry is and how this process could help treat cancer and acne? Join me in this intriguing journey, but before getting into the details, it is important to understand a few fundamental principles!
The Power of Light and Photochemistry
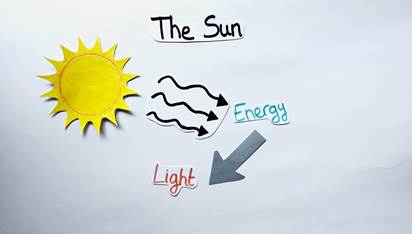
The sun is the source that makes life on our planet possible. It is a gigantic sphere of hot gas that produces a lot of energy appearing in the form of light (Figure 1). This light is essential and without it, the earth would be pretty barren since there would be no plants, animals or humans.
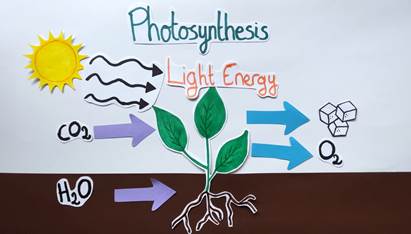
Photosynthesis is a striking example of the power of the sun. This natural photochemical process enables plants to capture light energy from the sun and transform it into chemical energy in the presence of carbon dioxide (CO₂) and water (H₂O). Plants can synthesize a sugar called glucose while releasing oxygen (O₂) (Figure 2). Thanks to that, they can grow and also, we can breathe! If plants can use light energy to carry out chemical reactions, why can’t chemists use this type of energy on molecules in the laboratory?
E-Geometry vs Z-Geometry: Minor Modification BUT Crucial Impact
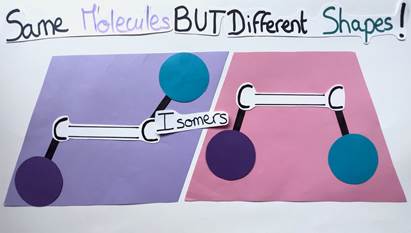
Before we return to the question of whether and how chemists may use light energy, let’s take a little excursion: Have you ever heard of molecules called alkenes? Probably not, as they are not well known, but such molecules are widely prevalent in natural and bioactive products! Basically, an alkene is a planar molecule composed of two carbon atoms linked by a double bond. Each carbon atom can carry a chemical group. A special feature of alkenes is that they can have different geometries depending on the arrangement of their chemical groups. If the groups are on opposite sides of the double bond, the alkene is said to have an E geometry (from the German word “entgegen” meaning “opposite”). Conversely, if the groups are on the same side of the double bond, the alkene has a Z geometry (from the German word “zusammen” meaning “together”) (Figure 3).
It’s like comparing different shapes: The E alkene is diamond-shaped while the Z alkene is trapezoidal. Although these molecules are similar, they differ in their geometry and are therefore called isomers (Figure 4). The aforementioned geometry of alkenes is very important, as it can impact the molecule’s chemical properties and functions.
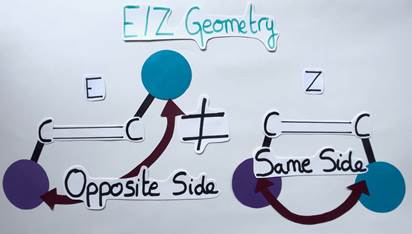
For a better understanding, let’s take a look at a concrete example. Aliretinoin is a molecule possessing several alkenes. It is used in the treatment of cancer. If the geometry of two alkene units is reversed, another isomer called Isoretinoin is obtained. In this case, the molecule is used to treat acne (Figure 5). It is therefore essential to be able to control the geometry of alkenes. But how can chemists do that?
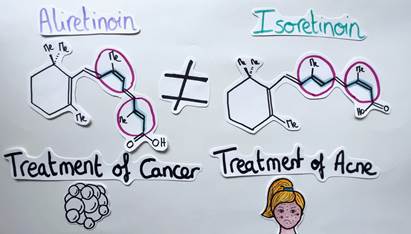
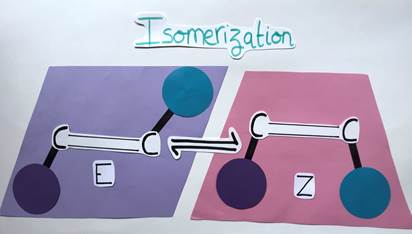
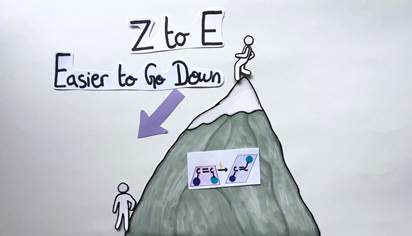
Isomerization of Alkenes: Climbing vs Going Down a Mountain
Let’s take another look at our E and Z alkenes. The process of switching from one to the other is called isomerization (Figure 6). Since these two molecules are quite similar, you might think it would be easy to go from one geometric form to the other but in reality, it’s way more complex…
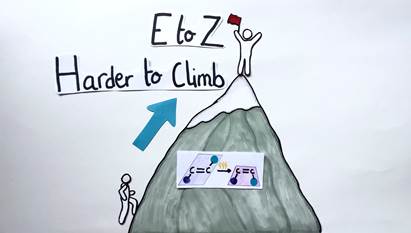
In general, it’s easy to convert a Z alkene to an E alkene, since in the E geometric form, the chemical groups are far apart and don’t interfere with each other. As a consequence, the process requires only very little energy. Conversely, it’s more difficult to convert an E alkene to a Z alkene, since a lot of chemical energy needs to be introduced into the system to force the chemical groups on the same side.
To visualize this concept, let’s picture a mountain. The E to Z isomerization would be symbolized by a person climbing up the mountain. The person’s body needs a lot of energy to reach the top (Figure 7). The Z to E isomerization would be represented by the same person going down the mountain. This time, the person’s body needs less energy and effort to reach the bottom (Figure 8).
Photochemistry as an Ideal & Sustainable Tool To Control the Geometry of Alkenes
This is where photochemistry comes into play! This term is used to describe chemical reactions induced by the energy provided by light. Indeed, in this case, the reactant can get energy from photons which can be considered as tiny particles of light. This branch of chemistry is very attractive because photochemical processes are greener and safer than thermal reactions in which the reactant needs heat as an energy source. In addition, photochemistry can provide access to compounds that are not accessible by classical thermal activation since it proceeds differently.
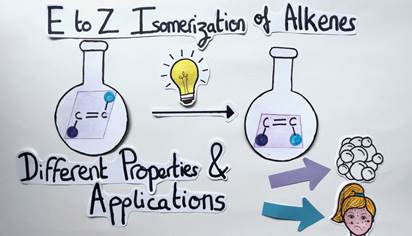
As a reminder, the E to Z isomerization of alkenes is a difficult chemical transformation because it requires a lot of energy. To solve this problem, like plants and the process of photosynthesis, my role as a chemist consists in harvesting light and converting it into chemical energy to allow this isomerization to happen (Figure 9).
Thanks to this process, the geometry of alkenes can be controlled and it is therefore possible and easier to modulate their chemical properties and applications. It’s a long and bumpy road ahead, but in this way, we might be able to treat cancer and acne with the same molecule by simply switching the alkene’s geometry!
For more insight into why it is crucial to control the geometry of a molecule, check out another blog post by one of our former fellows, Dr. Louise Ruyet, whose research focuses on 3D fluorinated molecules.
